
PRODUCT DESCRIPTION
- The mechanism of action for contraception is thickening of the cervical mucus that inhibits the passage of sperm.
- The mechanism of action for treatment of heavy menstrual bleeding is thinning of the uterine lining.
Please refer to the approved product labeling in your country for the approved duration of use, as the 8-year duration of use may still be pending approval in some countries.
KEY CLINICAL CHARACTERISTICS
Highly effective long-acting reversible contraceptive
About 99% effective at preventing pregnancy for up to eight years
Studied in a broad range of women
- 1,751 women ages 16-45
- 58% nulliparous (having never given birth)
- BMI range 16-62 and average 27
Effective at treating heavy menstrual bleeding
Effective treatment of heavy menstrual bleeding for 80% of women over 6 months, reducing menstrual blood loss by 91% after 3 months of use and by 96% after 6 months
Same-day insertion is possible
- Can be inserted at any time, if the provider is reasonably certain the woman is not pregnant
- Same day insertion is possible for patients without clinical evidence of infection, if indicated based on standard screening guidelines
AVAILABILITY
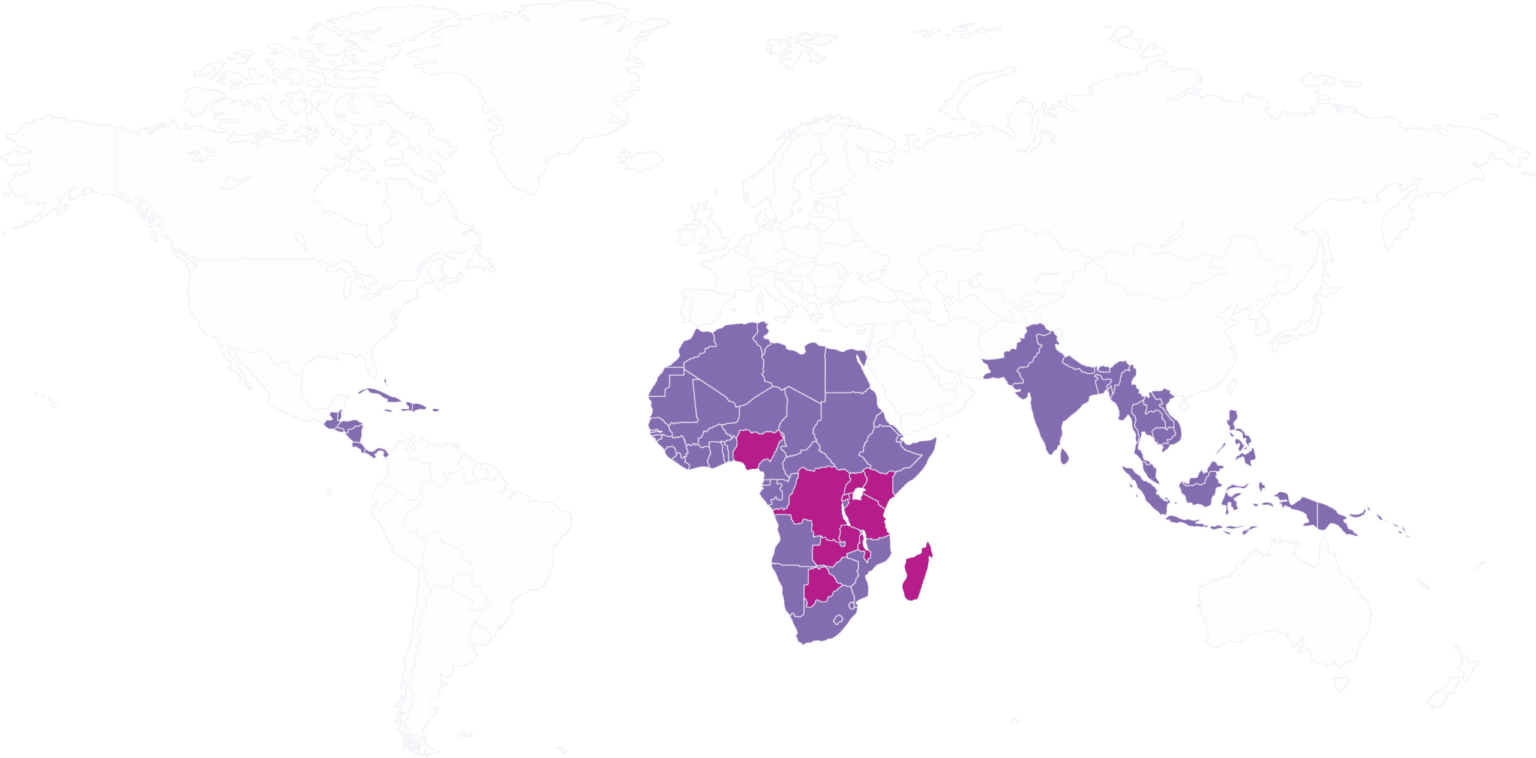
AVIBELA can be made available in the 88 countries shown in the map below. AVIBELA is currently registered in Botswana, the Democratic Republic of Congo, Kenya, Madagascar, Malawi, Nigeria, Rwanda, Tanzania, Uganda, and Zambia.
Available
Registered
HOW TO ACCESS AVIBELA
If you have questions about AVIBELA, please use the Contact Us form.
Contact Us
ADDITIONAL RESOURCES
*Please refer to the approved labeling for AVIBELA in your country.
IMPORTANT SAFETY INFORMATION
AVIBELA Important Safety Information
Indications
AVIBELA is a sterile, levonorgestrel-releasing intrauterine system indicated for contraception for up to 8 years; replace after 8 years if continued use is desired. AVIBELA is indicated for treatment of heavy menstrual bleeding (HMB) for up to 5 years; replace after 5 years if continued use is desired. AVIBELA may be particularly useful in women with HMB requiring (reversible) contraception.
Who is not appropriate for AVIBELA
AVIBELA is contraindicated when one or more of the following conditions exist: pregnancy; for use as post-coital contraception (emergency contraception); congenital or acquired uterine anomaly, including leiomyomas, that distorts the uterine cavity and would be incompatible with correct intrauterine system (IUS) placement; acute pelvic inflammatory disease (PID); postpartum endometritis or infected abortion in the past 3 months; known or suspected uterine or cervical malignancy; known or suspected breast cancer or other hormone-sensitive cancer, now or in the past; uterine bleeding of unknown etiology; untreated acute cervicitis or vaginitis, including bacterial vaginosis, known chlamydial or gonococcal cervical infection, or other lower genital tract infections until infection is controlled; acute liver disease or liver tumor (benign or malignant); conditions associated with increased susceptibility to pelvic infections; a previously inserted IUS that has not been removed; a history of hypersensitivity reaction to any component of AVIBELA.
Clinical considerations for use of AVIBELA
Use AVIBELA with caution after careful assessment if any of the following conditions exist and consider removal of AVIBELA if any of them arise during use: coagulopathy or use of anticoagulants; migraine, focal migraine with asymmetrical visual loss, or other symptoms indicating transient cerebral ischemia; exceptionally severe or frequent headache; marked increase of blood pressure; or severe arterial disease such as stroke or myocardial infarction. Consider removal of AVIBELA if the any of the following conditions arise during use: uterine or cervical malignancy, or jaundice. Because irregular bleeding/spotting is common during the first months of AVIBELA use, exclude endometrial pathology (polyps or cancer) prior to the insertion of AVIBELA in women with persistent or uncharacteristic bleeding. If the threads are not visible or are significantly shortened, they may have broken or retracted into the cervical canal or uterus. If AVIBELA is displaced (e.g., expulsed or perforated the uterus), remove it.
Pregnancy related risks with AVIBELA
If pregnancy should occur with AVIBELA in place, attempt to remove AVIBELA because leaving it in place may increase the risk of spontaneous abortion and preterm labor. Removal of AVIBELA or probing of the uterus may also result in spontaneous abortion. Evaluate for ectopic pregnancy because the likelihood of a pregnancy being ectopic pregnancy is increased. Tell people about the signs of ectopic pregnancy and associated risks, including loss of fertility. People with a history of ectopic pregnancy, tubal surgery, or pelvic infection have a higher risk of ectopic pregnancy.
Educate about PID or endometritis
Insertion of AVIBELA is contraindicated in the presence of known or suspected PID or endometritis. IUSs have been associated with an increased risk of PID, most likely due to organisms being introduced into the uterus during insertion. In the contraception study, one person diagnosed with PID and two people diagnosed with endometritis developed the infection within a week of insertion. One endometritis case was diagnosed at 39 days after insertion. The remaining 11 cases of PID and endometritis were diagnosed more than 6 months after insertion, including one at 30 days after IUS removal. In the HMB study, one person was diagnosed with PID about 5 months after insertion. Counsel people who use AVIBELA to notify a healthcare provider if they develop lower abdominal or pelvic pain, fever, chills, unusual or malodorous discharge, unexplained bleeding, genital lesions or sores, or dyspareunia. PID and endometritis are often associated with sexually transmitted infections (STIs); AVIBELA does not protect against STIs, including HIV. PID or endometritis may be asymptomatic but still result in tubal damage and its sequelae. Inform people about the possibility of PID or endometritis and that these infections can cause tubal damage leading to ectopic pregnancy or infertility, or infrequently can necessitate hysterectomy, or cause death.
Expect changes in bleeding patterns with AVIBELA
Spotting and irregular or heavy bleeding may occur during the first 3 to 6 months. Periods may become shorter and/or lighter thereafter. Cycles may remain irregular, become infrequent, or even cease. Consider pregnancy, including ectopic pregnancy, if menstruation does not occur within 6 weeks of the onset of previous menstruation. If a significant change in bleeding develops during prolonged use, conduct diagnostic tests to assess possible endometrial pathology.
Be aware of other serious complications and most common adverse reactions
Some serious complications with IUSs like AVIBELA are sepsis, perforation, and expulsion. Severe infection or sepsis, including Group A streptococcal sepsis (GAS), have been reported following insertion of other LNG-releasing IUSs. Aseptic technique during insertion of AVIBELA is essential to minimize serious infections such as GAS.
Perforation (total or partial, including penetration/embedment of AVIBELA in the uterine wall or cervix) may occur, most often during insertion, although the perforation may not be detected until sometime later. Perforation may also occur at any time during use. Perforation may reduce contraceptive efficacy. If perforation is suspected, locate and remove AVIBELA as soon as possible. Surgery may be required. Delayed detection or removal of AVIBELA in case of perforation may result in migration outside the uterine cavity, adhesions, peritonitis, intestinal perforations, intestinal obstruction, abscesses, and erosion of adjacent viscera. The risk of perforation is increased if inserted in patients who have fixed retroverted uteri, are postpartum, or are lactating. Delay AVIBELA insertion a minimum of 4 weeks or until uterine involution is complete following a delivery or a second-trimester abortion.
Partial or complete expulsion of AVIBELA may occur, resulting in the loss of contraceptive protection. Expulsion risk is increased when inserted immediately after delivery; it appears to be increased with insertions after second-trimester abortion, based on limited data. Risk of expulsion is increased for people with a history of HMB or greater than normal BMI at the time of insertion. Remove a partially expelled AVIBELA. If expulsion has occurred, a new AVIBELA may be inserted when there is reasonable certainty the person is not pregnant.
Ovarian cysts may occur and are generally asymptomatic. Cysts may be accompanied by pelvic or abdominal pain or dyspareunia. Evaluate persistent ovarian cysts.
In the AVIBELA contraception study, the most common adverse reactions (≥10% users) were vulvovaginal mycotic infections, vaginal bacterial infections, acne, nausea or vomiting, abdominal discomfort or pain, and procedural bleeding). In the AVIBELA HMB study, the adverse reaction profile was consistent with the adverse reaction profile in the contraception study.
Teach people to recognize and immediately report signs or symptoms of the aforementioned conditions. Consider evaluating people 4 to 6 weeks after AVIBELA insertion and during routine care, or more often if clinically indicated. Check threads during each evaluation.

